Research in the Tzelepis lab, in collaboration with scientists from the Wellcome Sanger Institute and Harvard University, has identified a protein that plays a key role in transforming normal tissue to cancerous, as a possible target for drug development. The research, published today in Molecular Cell, provides strong evidence that developing drugs that block the RNA-modifying protein known as METTL1 could give people with aggressive brain, blood, skin and kidney cancers new treatment options.
Dr Konstantinos Tzelepis said “This study provides another great example of what is possible with the use of CRISPR technologies and how we can take and prioritise precise genetic information and turn it into something of potential clinical benefit. Targeting RNA-modifying proteins can effectively destroy cancer cells and we hope that this research will provide the evidence necessary for drugs to be developed that target METTL1, potentially providing a new therapy against aggressive cancers with clear and unmet therapeutic need.”
Abstract from the paper
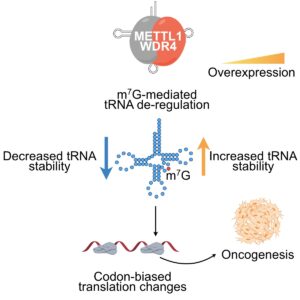
The emerging “epitranscriptomics” field is providing insights into the biological and pathological roles of different RNA modifications. The RNA methyltransferase METTL1 catalyzes N7-methylguanosine (m7G) modification of tRNAs. Here we find METTL1 is frequently amplified and overexpressed in cancers and is associated with poor patient survival. METTL1 depletion causes decreased abundance of m7G-modified tRNAs and altered cell cycle and inhibits oncogenicity. Conversely, METTL1 overexpression induces oncogenic cell transformation and cancer. Mechanistically, we find increased abundance of m7G-modified tRNAs, in particular Arg-TCT-4-1, and increased translation of mRNAs, including cell cycle regulators that are enriched in the corresponding AGA codon. Accordingly, Arg-TCT expression is elevated in many tumor types and is associated with patient survival, and strikingly, overexpression of this individual tRNA induces oncogenic transformation. Thus, METTL1-mediated tRNA modification drives oncogenic transformation through a remodeling of the mRNA “translatome” to increase expression of growth-promoting proteins and represents a promising anti-cancer target.
Read the full article here.